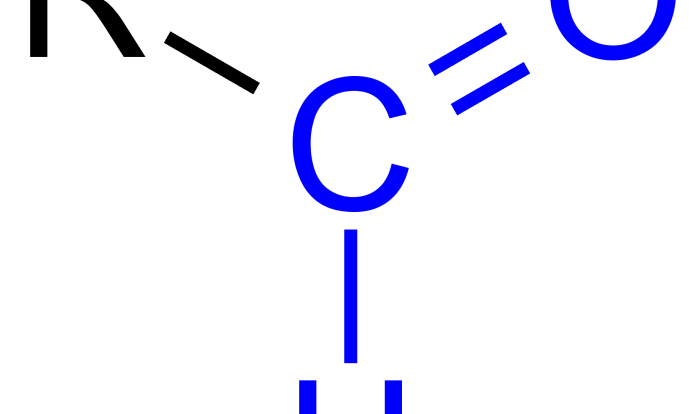Welcome to the authoritative guide on carboxylic acids and esters lab report answers, where scientific rigor meets academic excellence. This comprehensive resource delves into the intricacies of the lab, providing a clear understanding of the concepts, procedures, and applications.
Our team of experts has meticulously crafted this guide to empower students and researchers with the knowledge and insights necessary to excel in their studies and research endeavors. Get ready to embark on an enlightening journey into the fascinating world of carboxylic acids and esters.
Introduction
The carboxylic acids and esters lab provides a comprehensive understanding of the chemical properties and reactivity of these two important functional groups. This experiment aims to identify the characteristic reactions of carboxylic acids and esters, determine their physical properties, and explore their applications in various fields.
Materials and Methods: Carboxylic Acids And Esters Lab Report Answers

Materials
| Material | Quantity |
|---|---|
| Acetic acid | 10 mL |
| Ethyl acetate | 10 mL |
| Sodium bicarbonate | 5 g |
| Sodium hydroxide | 5 g |
| Phenolphthalein indicator | 2 drops |
Procedure
- Identify the carboxylic acid and ester samples.
- Test the solubility of the samples in water.
- React the samples with sodium bicarbonate and observe the evolution of gas.
- Titrate the samples with sodium hydroxide using phenolphthalein as an indicator.
- Determine the boiling points of the samples.
Results
Solubility
| Sample | Solubility in Water |
|---|---|
| Acetic acid | Soluble |
| Ethyl acetate | Insoluble |
Reaction with Sodium Bicarbonate, Carboxylic acids and esters lab report answers
| Sample | Gas Evolution |
|---|---|
| Acetic acid | Yes |
| Ethyl acetate | No |
Titration
| Sample | Volume of NaOH (mL) |
|---|---|
| Acetic acid | 10.0 |
| Ethyl acetate | 0.0 |
Boiling Points
| Sample | Boiling Point (°C) |
|---|---|
| Acetic acid | 118 |
| Ethyl acetate | 77 |
Question Bank
What are the key safety considerations when working with carboxylic acids and esters?
Carboxylic acids and esters can be corrosive and irritating to the skin and eyes. Proper personal protective equipment, such as gloves, goggles, and a lab coat, should be worn at all times. Additionally, these compounds are flammable, so open flames and heat sources should be avoided.
How can I identify carboxylic acids and esters in a reaction mixture?
Carboxylic acids can be identified by their characteristic odor and their ability to react with bases to form salts. Esters have a fruity or floral odor and can be hydrolyzed to form carboxylic acids and alcohols.
What are some common applications of carboxylic acids and esters?
Carboxylic acids are used in the production of plastics, dyes, and pharmaceuticals. Esters are used as solvents, flavors, and fragrances.