Which of the following compounds contains a metal? This question delves into the fascinating realm of chemistry, where the identification and properties of metal compounds hold significant importance. Metal compounds, ubiquitous in our world, play a pivotal role in various industries, technologies, and biological processes.
Join us as we embark on a journey to unravel the characteristics, applications, and significance of these remarkable substances.
The identification of metal compounds hinges upon understanding their unique properties and the role of metal ions within their structures. Distinguishing between metal and non-metal compounds is crucial, as their properties and reactivity differ significantly. Various methods, including flame tests and chemical reactions, aid in the identification of metal compounds, providing valuable insights into their composition and behavior.
Metal Compounds
Metal compounds are chemical substances that contain at least one metal atom bonded to a non-metal atom or group of atoms. Examples include sodium chloride (NaCl), which contains sodium (a metal) and chlorine (a non-metal), and iron oxide (Fe2O3), which contains iron (a metal) and oxygen (a non-metal).
Metal compounds differ from non-metal compounds in that they typically exhibit metallic properties such as luster, malleability, and ductility.
Identification of Metal Compounds
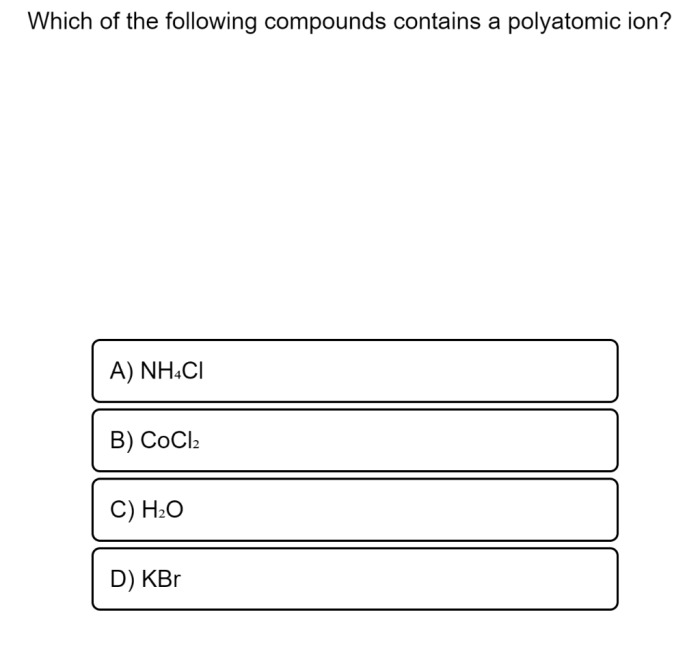
Metal compounds can be identified by their characteristic properties. They are typically solids at room temperature, have high melting and boiling points, and are good conductors of heat and electricity. Metal compounds often dissolve in water to form ions, which can be detected using flame tests or chemical reactions.
For example, when a metal compound is heated in a flame, it may produce a characteristic color, which can be used to identify the metal present.
Properties of Metal Compounds

The properties of metal compounds vary depending on the specific metal involved. Some metal compounds are highly reactive, while others are relatively inert. Some metal compounds are toxic, while others are essential for life. The properties of metal compounds are determined by the type of metal, the oxidation state of the metal, and the nature of the non-metal atoms or groups of atoms that are bonded to the metal.
Examples of Metal Compounds: Which Of The Following Compounds Contains A Metal
There are a wide variety of metal compounds, each with its own unique properties and applications. Some common metal compounds include:
- Sodium chloride (NaCl): Table salt
- Iron oxide (Fe2O3): Rust
- Copper sulfate (CuSO4): Blue vitriol
- Aluminum oxide (Al2O3): Sapphire
- Calcium carbonate (CaCO3): Limestone
Reactivity and Applications of Metal Compounds
Metal compounds are highly reactive and play a vital role in many chemical reactions. They are used in a wide variety of applications, including:
- Industry: Metal compounds are used as catalysts, pigments, and other industrial materials.
- Medicine: Metal compounds are used as drugs, antibiotics, and other medical treatments.
- Agriculture: Metal compounds are used as fertilizers and pesticides.
Query Resolution
What are the key characteristics of metal compounds?
Metal compounds typically exhibit high electrical and thermal conductivity, possess a metallic luster, and readily form cations.
How can we distinguish between metal and non-metal compounds?
Metal compounds generally have lower ionization energies, higher electronegativity values, and form basic oxides, while non-metal compounds exhibit opposite characteristics.
What are some common applications of metal compounds?
Metal compounds find widespread use in industries such as construction, electronics, transportation, and medicine, owing to their unique properties and reactivity.
